Introduction:
Golidocitinib is the first JAK1 selective inhibitor to enter into pivotal clinical development for the treatment of relapsed/refractory peripheral T cell lymphoma (r/r PTCL). Preliminary analysis of the JACKPOT8 study (NCT04105010) has shown promising antitumor efficacy and a manageable safety profile of golidocitinib. Here we reported the full analysis of the phase 2 multinational pivotal study JACKPOT8 Part B.
Methods:
This study enrolled r/r PTCL patients who had undergone at least one line of systemic therapies. All patients received golidocitinib at 150 mg once daily (QD) until disease progression or pre-defined discontinuation criteria were met. The primary endpoint was a CT-based objective response rate (ORR) assessed by an independent review committee (IRC) per Lugano 2014 criteria. Other efficacy endpoints included duration of response (DoR), progression-free survival (PFS), and overall survival (OS). The efficacy analysis set included patients whose pathological diagnosis of PTCL has been retrospectively confirmed by a central laboratory and who had at least one measurable lesion at the baseline assessed by IRC. The safety analysis set included all dosed patients.
Results:
A total of 88 efficacy evaluable patients with r/r PTCL were included in the analysis. The median age was 57.5 years, and the majority (64.8%) were male. The major pathological subtypes included PTCL NOS (56.8%), AITL (18.2%), and ALCL (11.4%). At the baseline, 54.5% of patients had ECOG PS of 1, 52.3% had elevated LDH levels, and 21.6% had bone marrow involvement. The median prior lines of therapies were two. All patients had been treated with chemotherapies, 50% had been treated with histone deacetylase inhibitors, and 10.2% had received CD30 targeted therapy.
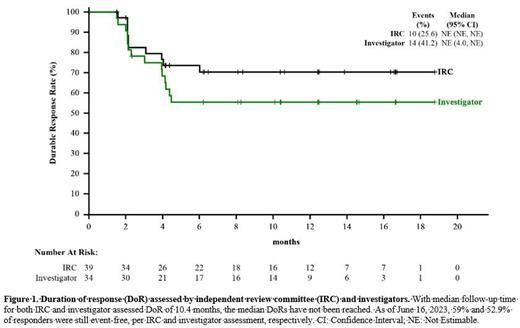
Per IRC assessment based on CT imaging, 39 patients achieved tumor response, with an ORR of 44.3% (95% CI: 33.7%, 55.3%), and 26 patients (29.5%) achieved complete response. The primary endpoint met the pre-defined target with statistical significance ( p < 0.0001). Tumor response was observed in various subtypes, irrespective of age, gender, baseline bone marrow involvement, and ECOG PS. With a median follow-up time of 10.4 months for DoR, 59% of responders were still event-free. The median DoR has not been reached, with an estimated 12-month DoR rate of 70.2%. With a median follow-up time of 9.6 months for PFS, the median PFS was 5.6 months. The longest PFS was 20 months, and the patient was still responding. Per investigator assessment, 34 patients achieved tumor response (ORR: 38.6%). The DoR and PFS by investigator assessment were similar to that of IRC assessed results.
With a median follow-up time of 15.1 months for OS, approximately 60% of patients were still alive, with an estimated median OS of 19.2 months.
A total of 112 patients were included in the safety analysis set. The median relative dose intensity was 100%. A total of 62 patients (55.4%) reported ≥ grade 3 treatment-emergent adverse events (TEAEs). The most common drug-related ≥ grade 3 TEAEs were hematological adverse events in nature, including neutropenia (25%), leukopenia (23.2%), and lymphopenia (18.8%). The majority of TEAEs were reversible and clinically manageable. Treatment-related AEs leading to dose interruption, reduction, and discontinuation were reported in 36.6%, 7.1%, and 8% of patients, respectively.
Conclusions:
This pivotal study met its primary objective, suggesting golidocitinib can be an effective treatment option with a manageable safety profile for patients with r/r PTCL. The updated data will be presented at the conference.
Disclosures
Mehta-Shah:Karyopharm Therapeutics: Consultancy; Celgene: Research Funding; Daiichi Sankyo: Consultancy, Research Funding; Kyowa Hakko: Consultancy; Janssen: Consultancy; Corvus Pharmaceuticals: Research Funding; C4 Therapeutics: Consultancy, Research Funding; Genentech/Roche: Research Funding; Bristol Myers-Squibb: Research Funding; AstraZeneca: Consultancy, Research Funding; Innate Pharmaceuticals: Research Funding; Ono Pharmaceuticals: Consultancy; Secura Bio/Verastem: Consultancy, Research Funding; Genentech: Consultancy. Yoon:Janssen: Consultancy, Honoraria, Research Funding, Speakers Bureau; Takeda: Honoraria, Speakers Bureau; Kirin Pharm: Honoraria, Speakers Bureau; Boryung: Research Funding; BMS: Honoraria, Speakers Bureau; Roche: Honoraria, Research Funding, Speakers Bureau; Pharos iBio: Consultancy; Beigene: Consultancy; Abclon: Consultancy; Samyang: Research Funding; Novartis: Consultancy, Honoraria, Speakers Bureau; GI cell: Consultancy; GC cell: Consultancy. Koh:Sanofi Genzyme: Research Funding; Proteina: Current holder of stock options in a privately-held company; Curocell: Current equity holder in private company; Deep Metrics: Current equity holder in private company; Novartis Korea: Consultancy; Takeda Korea: Consultancy; BMS Korea: Consultancy; Genome Opinion: Current Employment, Current equity holder in private company, Membership on an entity's Board of Directors or advisory committees; Tomocube: Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; Janssen Korea: Consultancy. Prince:Takeda: Speakers Bureau; Mallinkrodt: Speakers Bureau; Mundipharma: Speakers Bureau; Merck: Speakers Bureau. Yang:Dizal Pharmaceutical: Current Employment. Kim:Donga: Research Funding; Boryung: Research Funding; Sanofi: Research Funding; Roche: Research Funding; Kyowa-Kirin: Research Funding; Beigene: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal